The world is changing rapidly. Environmental, social, and corporate governance (ESG) metrics are motivating investment decisions globally. Meanwhile, plans to reduce greenhouse gas (GHG) emissions have only accelerated, with a growing number of governments putting net zero targets into law.
While transport electrification, increased renewables generation, and efficiency measures help reach these targets, emissions from heavy industries are much more challenging to abate. Heavy industry includes the manufacturing of steel, cement, glass, and chemicals together with bulk transport by ship and heavy goods vehicles.
A “hydrogen economy” has long been suggested to meet these challenges and, given recent research and tech development, provides a feasible path to achieving our collective net zero targets.
Introducing the hydrogen economy
The term hydrogen economy was first proposed by Lawrence Jones at the University of Michigan in 1970. His paper outlined a solution to link the growth of nuclear power to the transport sector in order to address air pollution and peak oil supply. At the time, the bulk manufacturing and cryogenic storage of hydrogen had recently been mastered to supply rocket fuel for space exploration, which inspired Jones to coin the term.
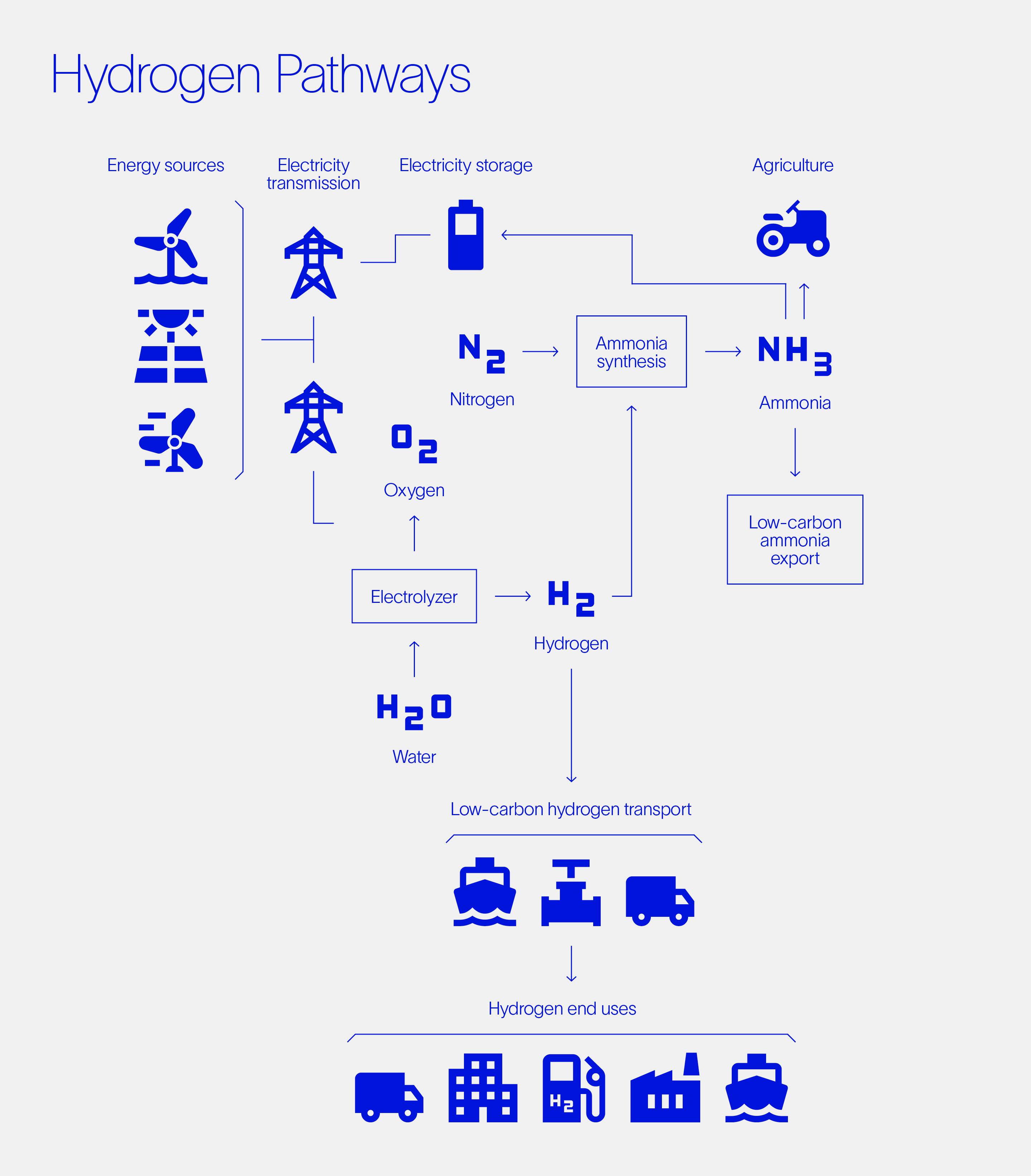
Today’s growing interest in hydrogen is motivated more by climate change than peak oil supply. The principles, however, are the same as those outlined in 1970 and include at their heart the concept of producing hydrogen via the electrolysis of water.
Unfortunately, the production of hydrogen is currently dominated by steam methane reforming (SMR) rather than electrolysis. The former is one of the most economic ways to produce the 94 million tons of hydrogen required, largely for ammonia plants and petroleum refineries, globally each year. But it is also a high-temperature process that produces carbon dioxide (CO2) as a by-product, resulting in SMR’s direct responsibility for hydrogen production currently accounting for over 1% of global GHG emissions.
Hydrogen’s various colors—and what they mean
The carbon footprint of hydrogen (or other chemicals) is commonly categorized using a color system. Hydrogen produced from the electrolysis of water is referred to as green hydrogen whereas SMR (given that it’s sourced by fossil fuels) produces grey hydrogen, The gas itself has no color, but the terms—rightly or wrongly—attempt to distinguish the environmental credentials of each type.
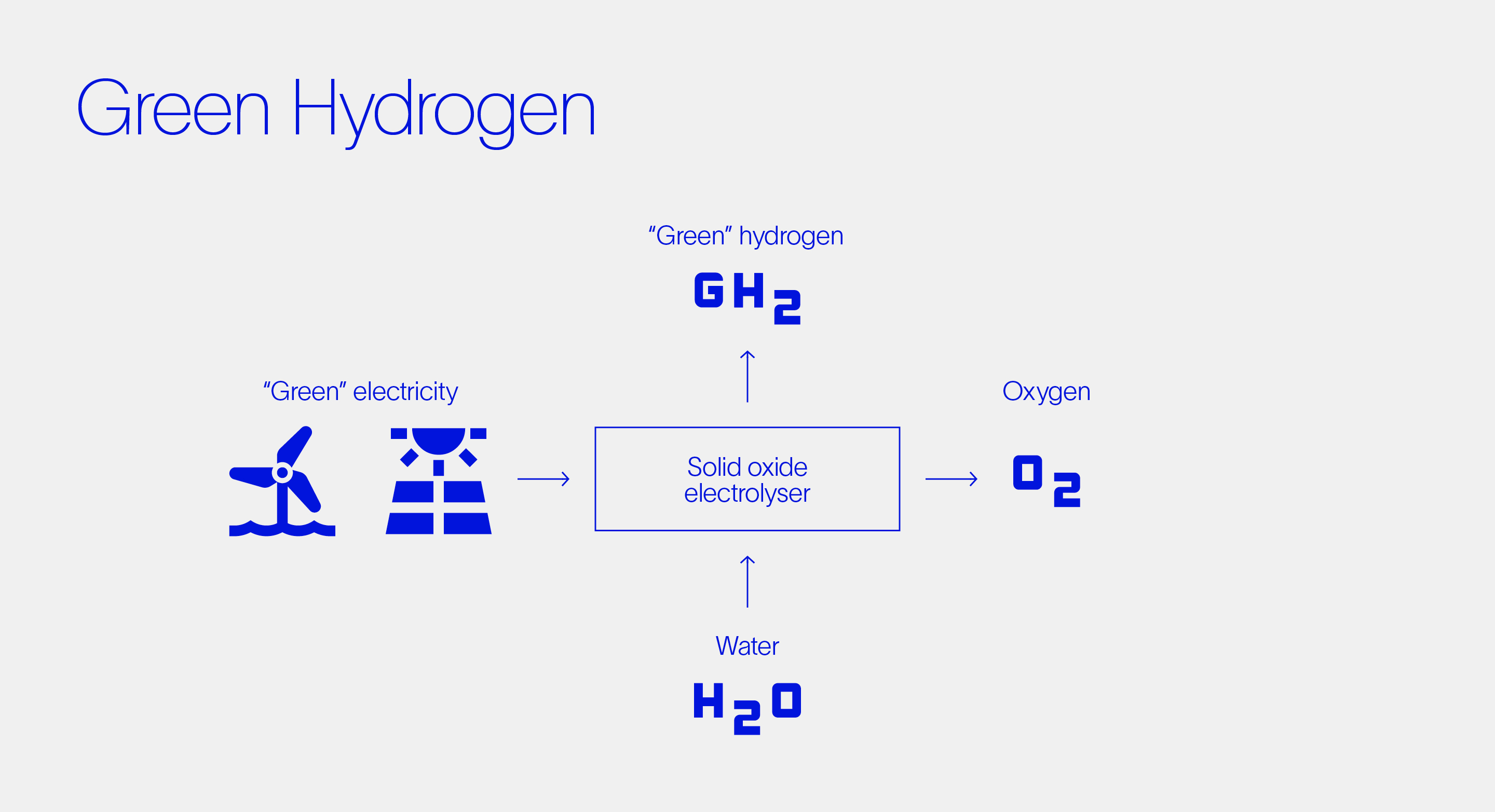
The being said, it’s important to note that the true carbon footprint of any product over its lifespan must be quantified in a detailed life cycle assessment. This assessment is dependent on many factors including specific application, geographic location, and end-of-life waste management.
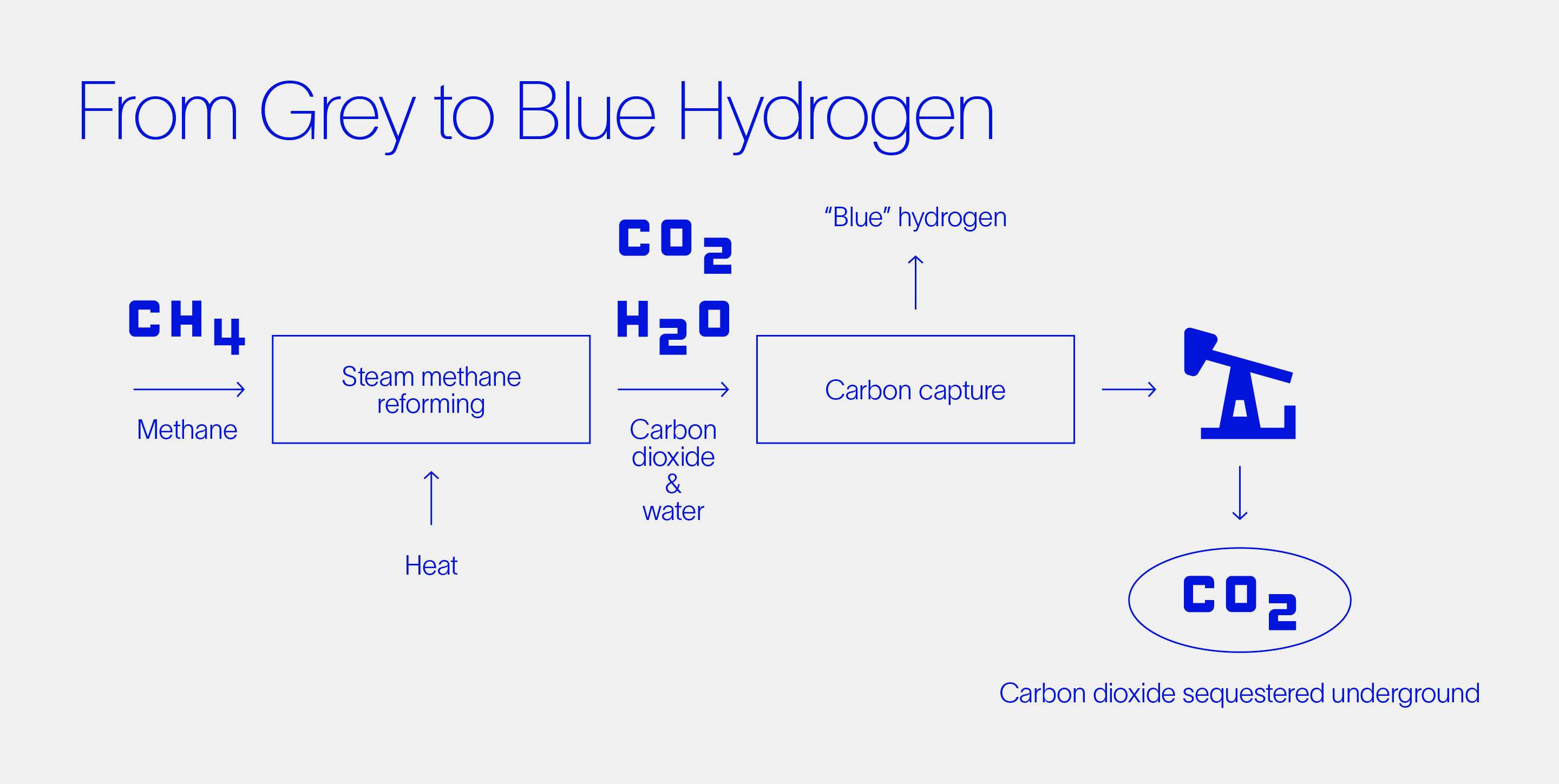
The carbon footprint of grey hydrogen can be abated by capturing the CO2 from the SMR process and sequestering it underground. SMRs that include a water-gas shift reaction produce gases of water and CO2 from which pure CO2 can then be easily extracted. Placing this CO2 safely in geological storage partially offsets the carbon footprint of the process, with the resulting product being referred to as blue hydrogen. In a vision to sequester vast quantities of CO2, Norway’s Northern Lights project aims to provide Europe with the CO2 shipping and sequestering networks it needs to achieve its decarbonization goals. Benefitting from this infrastructure, new hydrogen technology (such as ZEG’s) can then scale the production of blue hydrogen.
What about mass hydrogen production and storage?
Achieving net zero targets will ultimately demand the mass production of low-carbon fuels. Green hydrogen, for example, which is produced via electrolysis, provides a key electron-to-molecule bridge and has the lowest carbon footprint. This trend is further motivated by the increasing capacity of wind and solar generation, along with the need to store and transport this energy. Energy storage is critical for providing grid flexibility, limiting the need for expensive transmission line upgrades and wasteful curtailment of intermittent renewables.
While producing low-carbon hydrogen poses a technological challenge, so does its storage. Being the lightest element, hydrogen requires significant energy to liquefy at atmospheric pressure (-253°C). Alternately, cylinders can be used to store hydrogen at ambient temperatures and high pressures (up to 10,000 psi). The compression process is less energy intensive and costly than liquefication, although the volumetric density of the hydrogen is lower. (Hydrogen has a density of 71 kg/m3 as a liquid versus 42 kg/m3 as a gas at 10,152 psi.)
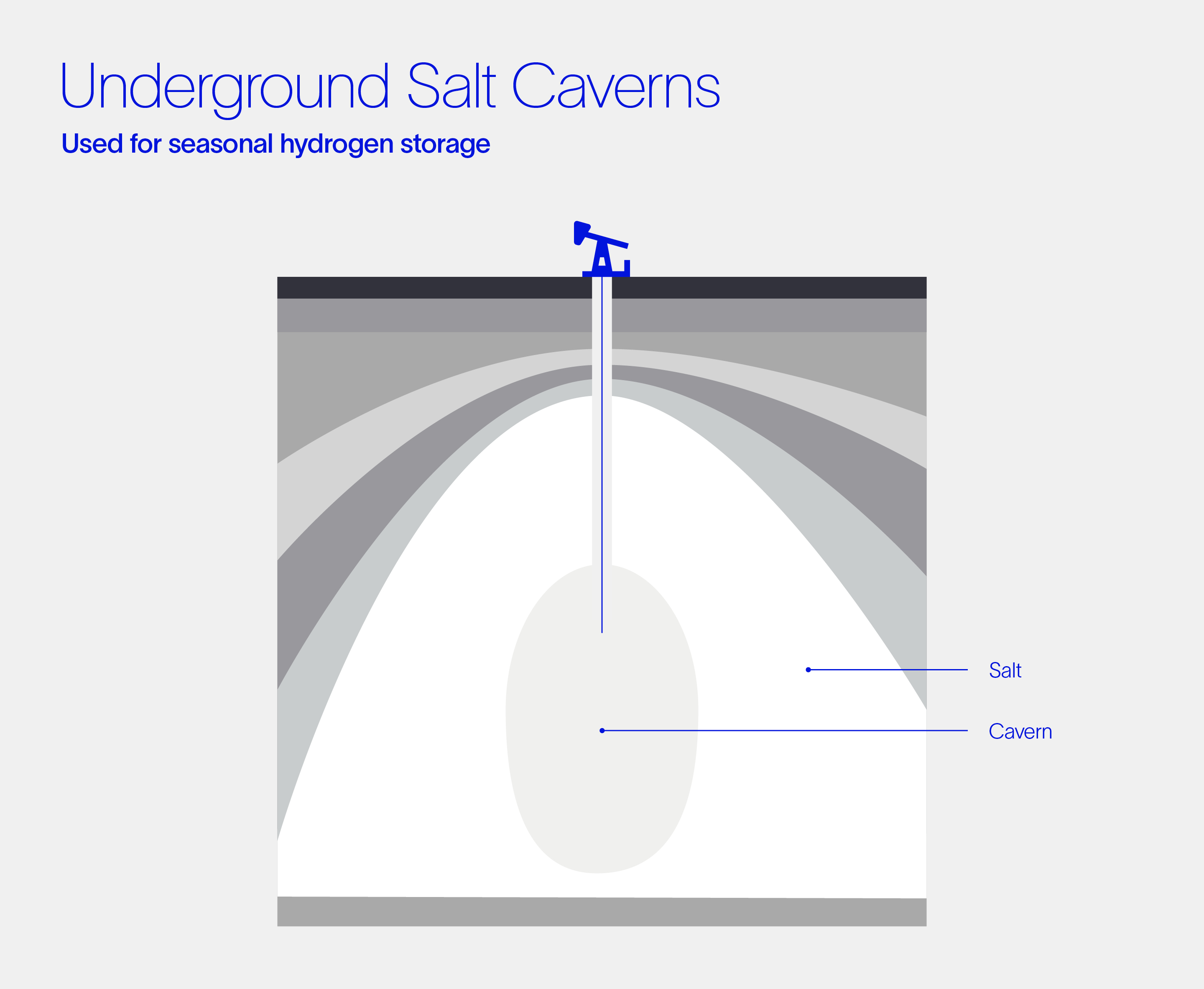
Subsurface salt caverns have been used for decades to store natural gas, and some facilities are currently used for hydrogen storage. Where can you find them, you might ask? Two hydrogen storage salt caverns have operated in Texas since the 1980s, with three smaller caverns in Teesside, United Kingdom.
Given the vast size of these caverns (10^5 – 10^6 m3), they represent the least expensive option for large-scale hydrogen storage. Salt caverns could enable seasonal hydrogen storage between the summer and winter months, greatly enhancing the value of solar and wind power generation.
Beyond the horizon
The hydrogen economy is diverse and complex, covering low-carbon alternatives for transport, heat, energy storage, and chemical feedstocks. One of the key chemicals currently requiring a hydrogen feedstock is ammonia. An approach called the Haber-Bosch process, primarily used to produce agricultural fertilizers, accounts for more than 85% of yearly ammonia production. Curiously, the Haber-Bosch process operates at similar temperatures to solid oxide electrolysis and has been identified for tight integration to efficiently produce low-carbon-footprint green ammonia.
Hydrogen can also be used with captured CO2 to produce carbon-neutral products. Methanol, diesel, and an array of polymers can be synthesized using what is called the Fischer-Tropsch process, for example. This provides a clear pathway to a circular economy where waste products are fully recycled.
The applications are numerous, available, and accessible. It’s up to us to implement and improve them.




