SELECT S
Hydrogen sulfide removal adsorbent
Mixed-metal-oxides-based adsorbents to remove harmful H2S from liquid and dry and water-saturated gas streams.

THIOPAQ® O&G* biodesulfurization system uses nonhazardous, naturally occurring, self-regulating bacteria to sweeten gas by converting H2S in a produced gas stream to manageable solid elemental sulfur. The process also includes extraction of the sulfur from the system, for use in a variety of agricultural applications or disposal in a landfill. It thus addresses two goals: environmental stewardship and positive economics.
The process system is highly efficient in converting hydrogen sulfide to sulfur. Compared with typical caustic scrubber systems, only modest amounts of caustic addition are required to maintain system alkalinity and pH for absorption. No heating equipment is used for regeneration and no catalyst is needed for conversions. This approach successfully reduces system cost. Small amounts of nutrients are added to the system to keep the bacteria in good health. After the one-time addition of bacteria to the system, the colony expands or contracts based on the feeding of H2S into the system. This enables the biological system to be self-regulating, supporting significant process turndown if conditions demand.
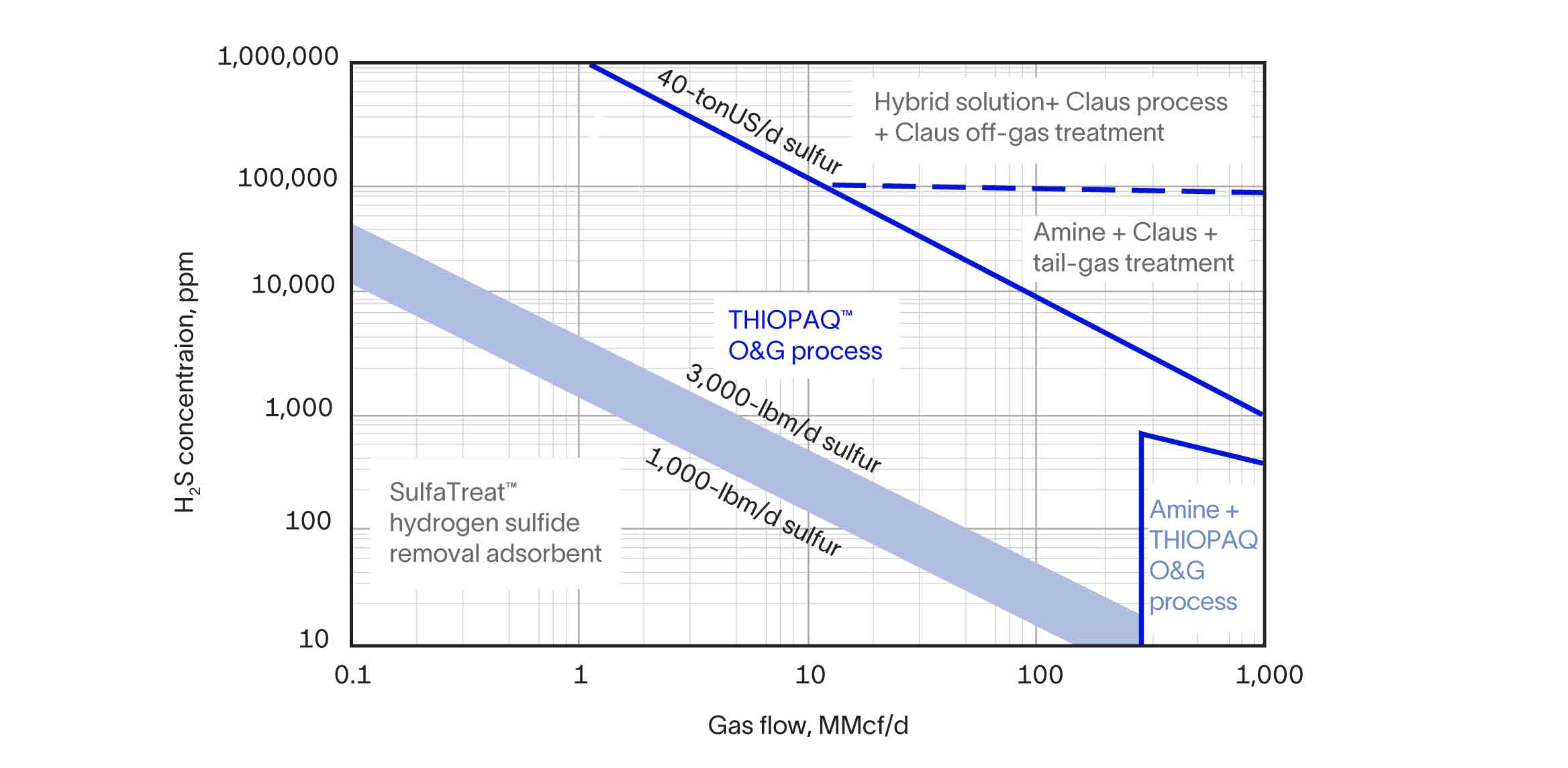
The process removes H2S from low-, medium-, and high-pressure streams in direct treat operations. It also easily installs for indirect treating and runs downstream of amine, Claus, and membrane units for emissions cleanup and sulfur recovery. The THIOPAQ O&G system produces treated gas that meets a typical H2S outlet specification of 4 ppm or less.
The process liquid that is home to the bacteria is regulated by a programmable logic controller (PLC), which monitors pH, conductivity, temperature, oxygen demand, and many other parameters. Should any of these parameters fall outside its operational range because of changes in gas flow rates, total sulfur loading, or both, the PLC automatically adjusts the parameter to ensure that the bacteria continue their highly efficient conversion cycles.
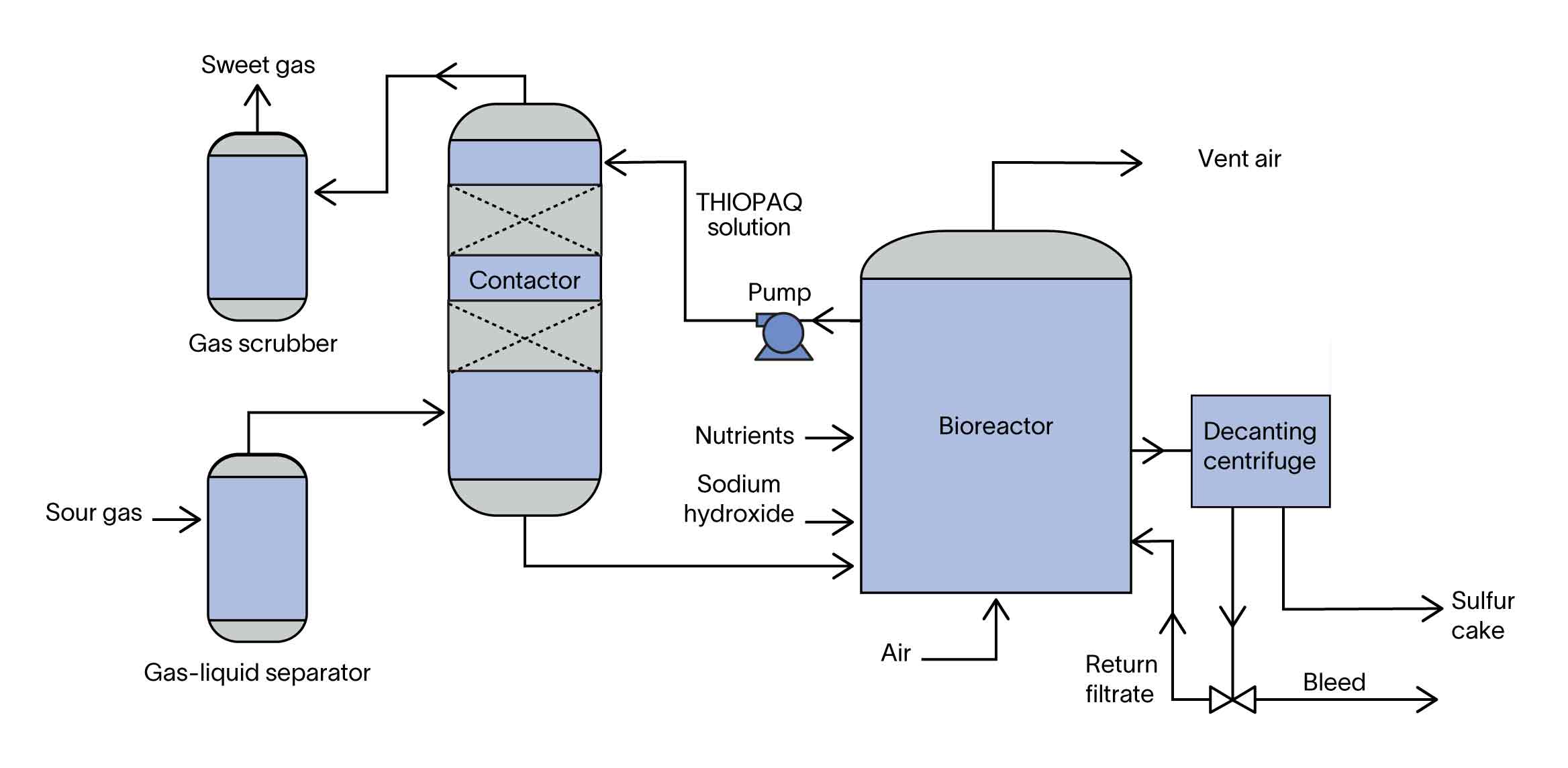
H2S absorption by aqueous soda solution
To begin, a pH-controlled aqueous soda solution (THIOPAQ solution) flows into a contactor containing plastic packing. Within the vessel, sour gas flows in a countercurrent direction to the THIOPAQ solution. The aqueous solution absorbs H2S from the natural gas stream, forming sulfide ions (HS–), and the sweetened gas stream exits the contactor with as much as 99% of the H2S removed. The contactor is the only place where H2S is present in the process other than in some gas inlet scrubbing vessels that are required upstream to better clean the gas for improved H2S removal.
Conversion of hydrogen sulfide ions into elemental sulfur
The second part of the process is conversion of the hydrogen sulfide ions that formed in the process solution. This conversion occurs when the solution is pumped into bioreactors where the haloalkaliphilic strains of the Thioalkalivibrio bacteria are stimulated by oxygen delivered by variable-speed air blowers. The bacteria oxidize and covert the sulfide ions into elemental sulfur. An enzyme is produced during the biological conversion that covers the sulfur particles, making them nonsticky or hydrophilic. This is unlike other liquid processes, which require chemicals for conversion and produce sticky or hydrophobic sulfur. Because sulfur particles are present throughout the process wherever the liquid solution exists, it follows that nonsticky sulfur particles lead to significantly less downtime for plant maintenance.
Removal of sulfur particles
The final step is removal of the converted sulfur particles. Testing and experience have shown that compared with vacuum filters and filter presses, the decanting centrifuge is the most efficient and cost-effective method of removing sulfur particles. The supernatant liquid is returned to the system to minimize requirements for additional fluids.
THIOPAQ is a registered trademark of Paques BV.
* THIOPAQ O&G is a technology owned by Paqell BV, a joint venture of Shell International and Paques BV.

THIOPAQ O&G biodesulfurization system is part of our Transition Technologies™ portfolio. Learn about our collaborative approach and full field development solutions that drive high performance while reducing environmental impact throughout the life of your asset.
Read More